
It represents the steady-state heat flow through a unit area of a material resulting from a temperature. It is a fundamental property, independent of the quantity of material. Read this K-value off the chart (approximately 21.3). A k-value (sometimes referred to as a k-factor or lambda value ) is a measure of the thermal conductivity of a material, that is, how easily heat passes across it. Note where the line crosses the methane axis.Connect the points with a straight line.On the right-hand vertical axis, locate and mark the point containing the temperature 60☏.On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia.Example įor example, to find the K value of methane at 100 psia and 60 ☏. S e l e c t o n e o f t h r e e h y d r o c a r b o n s: m e t h a. (A) Make a DEW P calculation for T 343.15 K. K-v a l u e) f o r a s e r i e s o f h y d r o c a r b o n s.

Many DePriester charts have been printed for simple hydrocarbons. Answer to Assuming the validity of the De Priester charts, make the following. "K" values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between. These nomograms have two vertical coordinates, one for pressure, and another for temperature. DePriester in an article in Chemical Engineering Progress in 1953. The average absolute error between experimental and predicted K-values for the new model was 4.355% compared to 20.5% for the Almehaideb correlation, 76.1% for the Whitson and Torp correlation, 84.27% for the Wilson correlation, and 105.8 for the McWilliams correlation.DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature. Comparisons of results show that the currently published correlations give poor estimates of K-values for all components, while the proposed new model improved significantly the average absolute deviation error for all components. These K-values were then used to build the model using the Discipulus software, a commercial Genetic Programming system, and the results of K-values were compared with the values obtained from published correlations. Part 2: A refinery in Northwest Pennsylvania produces asphalt as an important product that brings revenue, particularly during the summer months.
DEPRIESTER CHART K VALUES SERIES
as the value for a series of hydrocarbons Select one of three hydrocarbons. K values for light hydrocarbons at high temperatures (DePriester Chart). Material balance techniques were used to extract the K-values of crude oil and gas components from the constant volume depletion and differential liberation tests for the oil and gas samples, respectively. This Demonstration applies a DePriester chart a set of nomograms to find the. Constant Volume Depletion (CVD) and Differential Liberation (DL) were conducted for these samples. In this paper, 732 high-pressure K-values obtained from PVT analysis of 17 crude oil and gas samples from a number of petroleum reservoirs in Arabian Gulf are used.
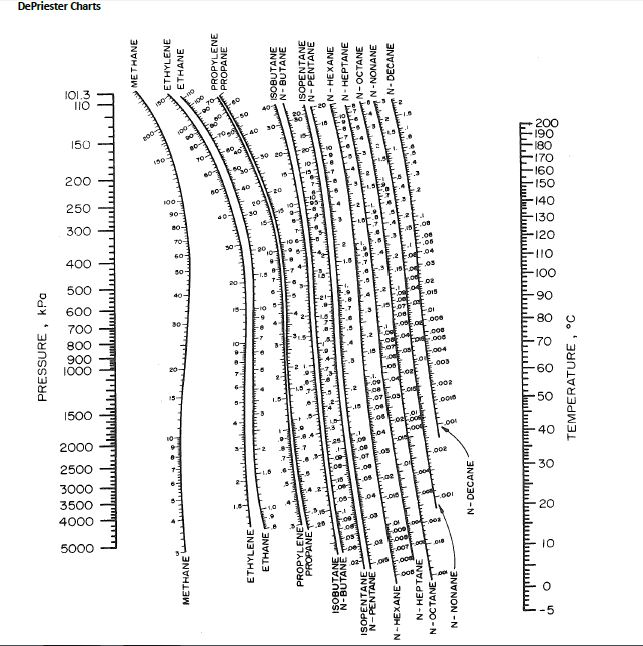
The new model is applied to multicomponent mixtures.
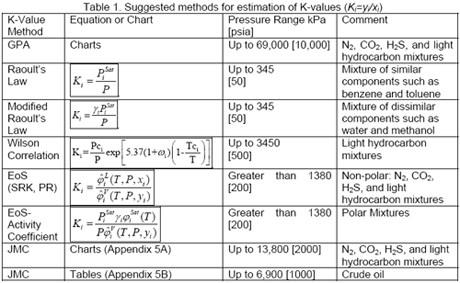
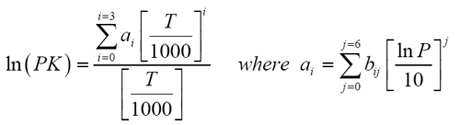
This paper presents a new model for predicting K values with genetic programming (GP). Several techniques are available in the literature to estimate the K-values. In particular, they are critical for reliable and successful compositional reservoir simulation. Drag or click the locator to display the values of the temperature and pressure as well as the -value for various light hydrocarbons. They are important in predicting compositional changes under varying temperatures and pressures in the reservoirs, surface separators, and production and transportation facilities. For light hydrocarbons, the approximate -values can be determined from DePriester charts, which have been fit to the following equation:, where the constants, ,, ,, and are tabulated 1. Equilibrium ratios play a fundamental role in understanding the phase behavior of hydrocarbon mixtures.


 0 kommentar(er)
0 kommentar(er)
